We use cookies to make your experience better.
TimmersGems has a new website, existing customers also need to register again.
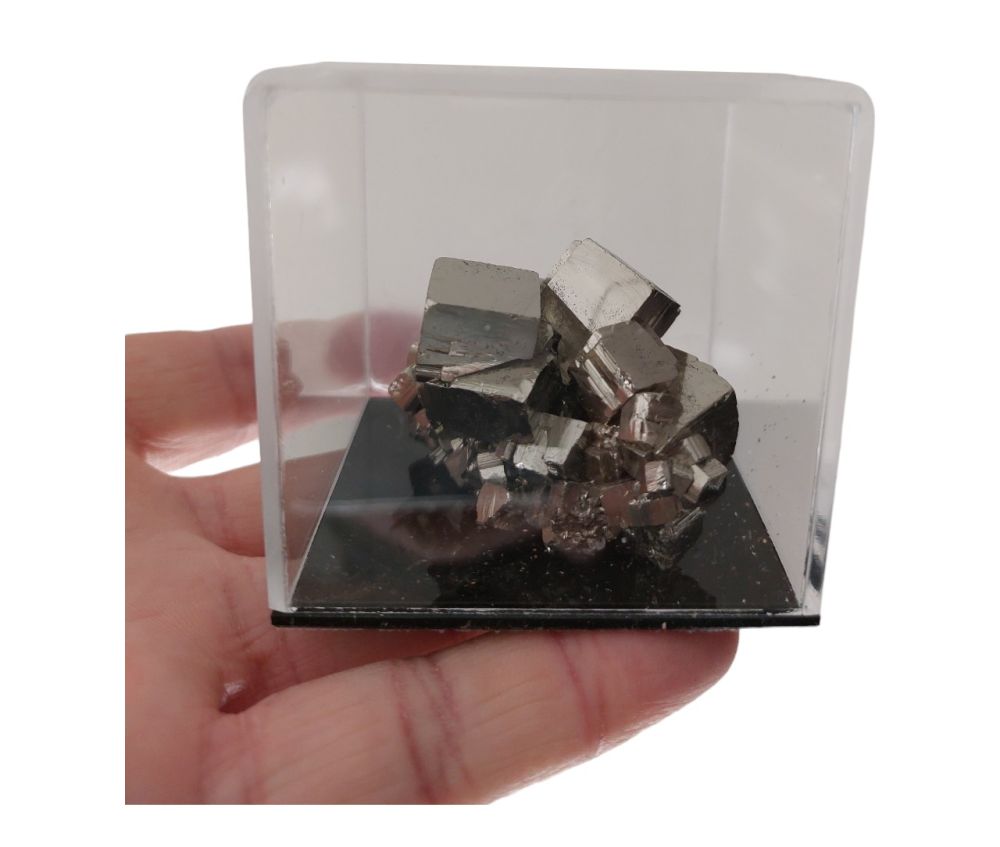
Pyrite (of museum quality) in a beautiful luxurious plexiglass box.
Fantastic quality Pyrite placed in protection to keep the mineral pure and prevent oxidation and discoloration.
Availability:
In stock
SKU
121498
- Buy 5 for €29.00 each and save 17%
Pyrite, also known as iron molar, is a mineral with the chemical formula FeS2, or iron disulfide. This mineral is an important source of iron and sulfur. It often crystallizes in well-formed euhedral cubes with characteristic striations and a goldish luster. It is also known as 'tinsel' or 'fool's gold' because it is sometimes confused with gold. The term 'cat's gold' is sometimes used incorrectly, but it actually refers to yellow mica or resin. Pyrite is common and can give the illusion of great wealth, but it is not very valuable, although it can be traded. It is found together with other sulfur-rich minerals and oxides, in quartz veins, sedimentary rocks, coal seams and as a substitute mineral in fossils. Pyrite is a disulfide with sulfur pairs (S2)2- and Fe2+ ions in an octahedral configuration and a t2g6 low spin state. Since both ions have a closed configuration, it is a diamagnetic semiconductor. When pyrite weathers, iron (hydr)oxides are formed and sulfuric acid is released. This acid can react with other minerals, leading to the formation of minerals such as gypsum, alunite and jarosite. A well-known location for pyrite is the island of Elba. The name 'pyrite' is derived from the ancient Indo-European word 'pyr' (fire), similar to 'pyrotechnics' (fireworks art) or the English 'pyre' (funeral pyre), because pyrite produces sparks when pressed against flint or iron beaten. Nowadays 'flint' means something else, namely a form of SiO2. Pyrite is also known as ferrous sulfide, sulfuric iron, and less commonly as iron blende and iron molar. Pyrite was sometimes sold as fake gold in the past because of its gold-like luster. However, this shine changes when the gloss surface is tilted, while the shine of gold remains the same. Pyrite is angular with sharp edges and hard, unlike gold, which is round and soft.
| Dimensions | Divers |
|---|---|
| Country of Manufacture | Peru |